只需50種藥就可以治療所有疾病?人工智能顯威力
|
在曼哈頓中城瑞典教會狹長的哥特式建筑中,丹尼爾·科恩在雅致而安靜的咖啡館里介紹了一番仿制藥后稍事休息。他走到靠近前門的老舊鋼琴前坐了下來,然后流暢而完美地彈奏了電影《綠野仙蹤》中的插曲《飛越彩虹》。
如果說人體生物學是科學領域里的復雜琴譜,那么科恩就是懂得如何彈奏它的行家。他曾經是法國非營利實驗機構Généthon的主導人物,后者在1993年12月繪制了歷史上的第一張人類基因組圖譜。可以說他是大數據和自動化進入基因研究的“引路人”,因為他和他的團隊率先證明了有可能用超高速計算機來加快DNA樣本的處理速度。
全世界科學家的工作都以科恩提出的思路為基礎,而作為免疫學專業醫學博士,他本人已經在研究者和醫藥公司高管的位置上取得了成功。然而,25年過去了,基因學幾乎沒能像許多初期創新者期望的那樣實現一些改變規則的醫學突破。今天,作為巴黎藥物初創企業Pharnext的首席執行官及創始人,科恩正在全力探究為何這道彩虹的末端不是一罐金子。
科恩離開鋼琴走回來,然后對我說:“人體內任何一種蛋白質都有很多功能,而不只是一種。就像你,作為個體你在人群中有很多種角色,而不只是一個。”科恩描述的現象就是“多效性”,即單個基因可以發揮多種作用,而且這些作用看起來都沒有關聯。這也是疾病復雜性的一個體現,而且它已經多次挫敗為最頑固疾病尋找治療方法的醫學研究者。
科恩不光了解多效性的意義,他還相信Pharnext等制藥公司也許很快就能對其加以利用,而且是在人工智能的有力協助下。科恩希望通過參悟人體的復雜性,并通過用人工智能來更有條理地分析并繪制疾病橫掃人體時的連鎖反應路徑來開發出適于眾多疾病的藥物組合。
科恩和他的團隊還在用人工智能來搜尋“老藥新用”帶來的治療方法,也就是將已有藥物組合起來,從而產生單一藥物不具備的療效。他們的長期目標是用機器學習來理順自己的后備藥品,使其效率遠遠超過大型制藥公司以緩慢著稱的研發部門。說到如何實現這一切,科恩的瞌睡眼就會因為激動而睜大。他說:“這很棒,而且很經濟。”
谷歌和IBM這樣的大企業以及Insilico Medicine、Recursion Pharmaceuticals和BenevolentAI等初創公司都在與Pharnext賽跑。它們都為人工智能工具投入重金,并用這些工具來分析數以百萬計的藥物樣本和患者數據,然后從中找出主要規律。但2007年成立的Pharnext領先大多數競爭對手好幾年,要是考慮到過去幾十年科恩率先在基因學和多效性方面進行的研究,它的優勢就更明顯了。
此外,十幾年來Pharnext在解決醫學難題時對人工智能的應用終于出現了關鍵拐點,這也許是最重要的。去年10月,該公司稱一組藥物的三期臨床試驗取得了積極成果。該組藥物代號為PXT3003,用于治療腓骨肌萎縮癥,這是一種罕見的神經退行性疾病,尚未找到治療方法。該病癥的主要誘因是某個基因的復制,而這樣的突變會引發一系列“下游”問題,從而使保護神經的施萬細胞退化為沒有保護作用的干細胞,最終導致神經突觸消失。這樣人就無法控制肌肉,進而出現肌肉萎縮。
據Pharnext介紹,上述三期臨床試驗結果(尚未得到同行審核)表明PXT3003不光能穩定病情,而且還有逆轉作用,證據是細胞開始再生。科恩指出,此前的療法只能延緩病情惡化的速度。在服用PXT3003后,統計數據顯示患者的兩項殘疾指標都有了明顯好轉。基于這些結果,美國食品與藥品監督管理局(FDA)在今年2月將FXT3003納入“快速通道”。這是一種加速審批程序,只有在FDA認為某種藥品在治療某種嚴重疾病方面展現出“卓越療效”時才會啟動。 |
In the elegant quiet of the café at the Church of Sweden, a narrow Gothic-style building in Midtown Manhattan, Daniel Cohen is taking a break from explaining genetics. He moves toward the creaky piano positioned near the front door, sits down, and plays a flowing, flawless rendition of “Over the Rainbow.”
If human biology is the scientific equivalent of a complicated score, Cohen has learned how to navigate it like a virtuoso. Cohen was the driving force behind Généthon, the French laboratory that in December 1993 produced the first-ever “map” of the human genome. He essentially introduced Big Data and automation to the study of genomics, as he and his team demonstrated for the first time that it was possible to use super-fast computing to speed up the processing of DNA samples.
Scientists worldwide have built on Cohen’s insights, and Cohen himself, an MD with a Ph.D. in immunology, has gone on to success as a researcher and pharma executive. But a quarter-century later, genomics has yielded few of the kinds of paradigm-changing medical breakthroughs that many of its early innovators hoped for. Today, as chief executive and founder of Paris-based drug startup Pharnext, Cohen is striving to understand why that rainbow hasn’t led to a pot of gold.
“Any protein in the body has many different functions, not only one,” he says, returning from the piano to talk with me, “just as you are a person who has many functions in the population, not just one.” The phenomenon Cohen is describing is “pleiotropy,” the capacity of a single gene to have multiple, seemingly unrelated effects. It is one of the complexities of disease that has repeatedly frustrated medical researchers in their quest for therapies for the most stubborn illnesses.
Cohen not only appreciates pleiotropy’s significance: He believes that Pharnext and other drugmakers may soon exploit it—with a powerful boost from artificial intelligence. By embracing the body’s complexity, and by using A.I. to more methodically analyze and map the way the chain reactions of disease sweep through the body, he hopes to develop combinations of drugs tuned to attack a plethora of medical conditions.
Cohen and his team are also applying A.I. to search for therapies that leverage “repurposing”—combining existing drugs in ways that give them therapeutic powers that each lacks in isolation. Their long-term goal is a drug pipeline that is far more efficient than Big Pharma’s notoriously slow R&D departments—streamlined by machine learning. Cohen’s sleepy gaze widens with enthusiasm when he describes how it’s all coming along. “Très bien,” he says. “Très économique.”
Running in the same race as Pharnext are companies ranging from giants like Google and IBM to startups such as Insilico Medicine, Recursion Pharmaceuticals, and BenevolentAI. All are deeply invested in the tools of A.I., using them to analyze millions of examples of drug and patient data and tease out patterns of significance. But Pharnext, founded in 2007, predates most of those competitors by several years—and has a longer head start when one factors in Cohen’s decades of earlier research in genomics and pleiotropy.
And perhaps most important, Pharnext’s application of A.I. to medical problems over the course of more than a decade has finally reached a critical inflection point. In October, Pharnext reported positive results for a Phase III trial in humans of one of its drug combinations. The compound is PXT3003, a treatment for a neurodegenerative condition called Charcot-Marie-Tooth disease (CMT), a rare disorder for which no cure has been found. The primary cause of CMT is duplication of a single gene, but a whole cascade of bad things ensues “downstream” from that mutation. Schwann cells, which protect nerves, regress into stem cells that don’t do their job. Axons in the nerves begin to die off. Muscles can’t be controlled, and they shrink as a consequence.
According to Pharnext, its Phase III results (which have not yet been peer-reviewed) showed CMT not merely stabilizing under PXT3003 but also being reversed, as cells began regenerating. Previous treatments, Cohen says, had managed only to slow patients’ decline. Under PXT3003, patients showed statistically significant improvement on two measures of disability. Based on those results, in February the U.S. Food and Drug Administration granted Pharnext “fast track” status for that therapy—an accelerated review process, awarded only when the agency thinks a drug demonstrates “superior effectiveness” in treating a serious disease. |
|
當然,這只是針對某一罕見病邁出的有希望的一步。但科技讓長期前景變得光明起來,它把藥物設計過程縮短了好幾年,讓Pharnext走上了捷徑。預臨床測試和臨床試驗通常需要8至10年,而從零開始開發新藥可能讓這個過程再延長7年,甚至更長時間。與之相反,就PXT3003而言,人工智能幫助Pharnext挑選了三種現有藥物進行再利用,包括治療肌肉痙攣和肌肉緊張的巴氯芬、解除阿片類藥物依賴的納曲酮以及用于降低血糖水平的緩瀉劑山梨糖醇。由于這些藥物都已經得到使用,Pharnext就可以跳過通常需要保證藥品安全性的一期臨床試驗,也就是那個“從零開始”的階段。
FDA的“快速通道”提高了PXT3003最早在2020年上市的可能性,而這只是Pharnext眾多項目中的一個。該公司很快就會啟動阿爾茨海默病治療藥物的第二次二期臨床試驗,以及肌萎縮側索硬化治療藥物的首次二期臨床試驗,這兩個項目也都采用了類似的老藥新用方法。
同樣重要的是,如果這些試驗取得成功,資金實力更強的效仿者將接踵而至。曾經在奧巴馬政府中擔任衛生及公共服務部部長的凱瑟琳·西貝利厄斯如今是多家醫療保健公司的顧問和董事。她認為這些工作是不斷增強的老藥新用趨勢的一部分。西貝利厄斯說:“所有這些都可能讓投資周期大幅縮短,而且有可能形成非常不同的定價水平,并為經濟動力一直不足的罕見病帶來許多可能性。這非常有吸引力。”哥倫比亞大學Kavli腦科學研究所的主任埃里克·坎德爾曾經獲諾貝爾生理學或醫學獎,他也是Pharnext的顧問。坎德爾認為Pharnext走在了這種趨勢的最前端,其方法“既有原創性,又很強大”。至于這樣的方法能否流行起來,坎德爾說:“我們很快就會知道。” |
It is, to be sure, only one hopeful step against one rare ailment. Still, technology has shortened Pharnext’s path in ways with promising long-term implications, shaving years off the drug-design timeline. Preclinical testing and clinical trials generally take eight to 10 years, and developing a novel drug completely from scratch can add seven years to the process, sometimes much more. In the case of PXT3003, in contrast, A.I. helped Pharnext select three existing drugs to repurpose: baclofen, a muscle relaxant; naltrexone, used to treat opioid dependence; and sorbitol, a glucose reduction used as a laxative. Because the drugs were already in use, Pharnext could skip the Phase I trials normally required to ensure their safety—and eliminate the “build from scratch” stage.
FDA fast-tracking increases the odds that PXT3003 could be on the market as soon as 2020—and it’s only one of Pharnext’s many projects. The company will soon begin a second Phase II trial of a drug with indications for Alzheimer’s and a first Phase II trial for an ALS therapy, in both cases using a similar repurposed combination.
Just as important: If these experiments succeed, copycats with deeper pockets could follow suit. Kathleen Sebelius, a secretary of Health and Human Services in the Obama administration who is now a consultant and board member for several health care companies, sees the efforts as part of a growing trend to repurpose. “That all leads to the possibility of a lot shorter investment cycle, and potentially a very different pricing point, and lots of possibilities for rare diseases where there just hasn’t been enough of a financial incentive,” she says. “And that has lots of appeal.” Eric Kandel of the Kavli Institute for Brain Science at Columbia University, a winner of the Nobel Prize in physiology or medicine who is an adviser to Pharnext, says that the startup is at the leading edge of the trend, calling its methodology “both original and powerful.” As for whether that approach will catch on widely, Kandel adds, “We should know soon.” |
****
|
在現代基因研究的“黎明”中,幾乎沒有人預見到疾病生物學的極端復雜性。當時,許多研究者都把基因視為人體的某種指導手冊。Celera Genomics的首席執行官及創始人克雷格·文特爾和美國國立衛生研究院的院長弗朗西斯·柯林斯等先驅人物被喻為“基因捕手”,這個稱號鼓勵著改革者們在全球各地搜尋那個“銀子彈”基因,也就是可以解釋并有助于治療某種疾病的基因。
從某個角度來說,這些研究者發現了真正的寶藏。舉例來說,基因學家南希·韋克斯勒花了好幾年時間在委內瑞拉拼湊亨廷頓病患者的族譜。這是一種罕見的遺傳疾病。她的工作讓人們發現可以通過某個基因的突變來預測一個人會不會患上這種疾病。
但科學家很快意識到基因圖譜不太像一本指導手冊,而更像是宜家家具隨附的那種配件目錄。此外,研究人員發現,其他“目錄”給基因和疾病的關系增添了復雜的變量,比如蛋白質組、DNA編碼蛋白、轉錄組以及所有將DNA轉換成蛋白質的核酸。
在“黎明”過后的“早晨”,失望帶來了真正的痛苦,因為研究人員認識到癌癥和阿爾茨海默病等復雜病癥并非由單個基因引起(就連已經確認誘發基因的亨廷頓病也依然無法治愈)。現在,科恩等人發現了簡化執念和藥物發現減少之間的聯系。藥物發現減少的具體表現包括FDA批準的新療法只有十分之一的成功率、螺旋式上升的藥物開發成本(塔夫茨大學最近的一項研究被稱為“26億美元的藥丸”)以及少量突破性新藥直線上升的價格,比如諾華制藥的白血病治療藥物Kymriah,它的每個療程的費用為47.5萬美元。
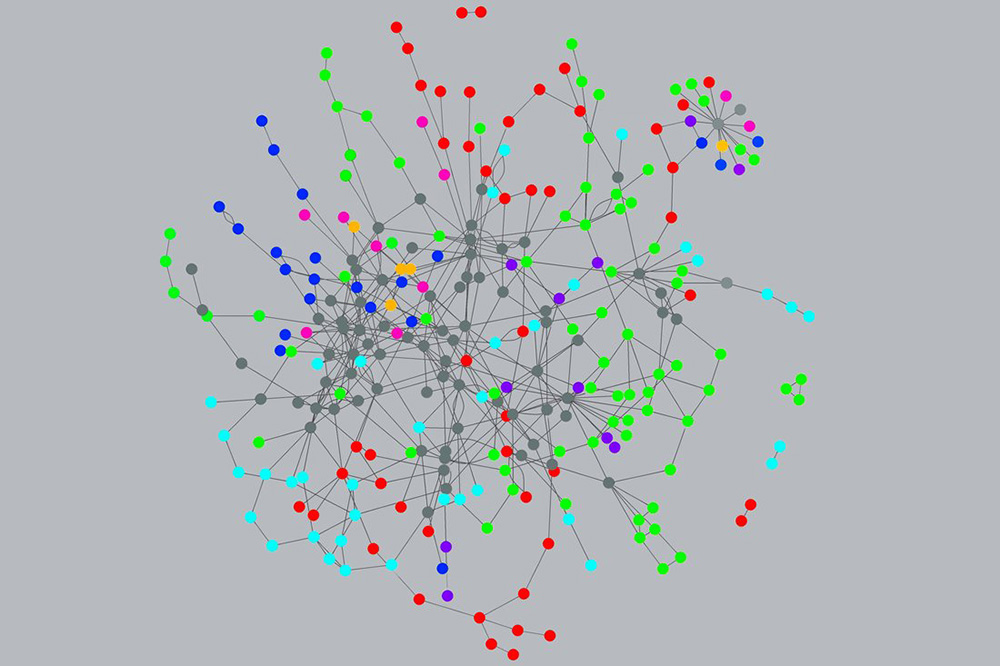
最近,研究人員開始在網絡科學的幫助下應付生物的復雜性。這門科學的首席傳播者是美國東北大學的教授艾伯特-拉斯洛·巴拉巴西。他在2014年出版的《鏈接》(Linked)一書讓一種觀念流行開來,那就是網絡理論可以對很多領域做出解釋,比如流行趨勢,比如性關系,再比如疾病。巴拉巴西等人意識到疾病就像一個壞信號,它通過基因-蛋白質-細胞-組織這樣的關系網絡傳播,直到所有這些“微小變化”展現出與疾病類似的癥狀。
復雜疾病是多種因素的共同作用,原因是多效性意味著任何蛋白質都可以在人體的不同部位發揮作用。Pharnext等初創公司認為藥物也有多效性,它們能同時對人體內一種以上的蛋白質產生影響,并進行一種以上的互動。為找到能夠應對復雜疾病的藥物組合,可以在數據中發現規律而且蘊含著巨大能力的機器學習必須與病理結構概念結合起來。
這就要求計算機科學家和生物學家的關系出現“進化”。較新的機器學習方法容納的數據遠超以往,它們還可以將信息分級,從而突破關聯這個框架。不過,讓這些“深度學習”神經網絡獲得預測能力需要建立一些漂亮的算法。
GNS Healthcare的 CEO及創始人科林·希爾就是這種算法的塑造者之一。這家設在馬薩諸塞州坎布里奇市的公司用了18年時間開發出名為REFS的計算機系統,意思是“逆向工程、正向模擬”(reverse engineering, forward simulation)。近年來,GNS Healthcare共籌集資金3800萬美元,投資方包括制藥巨頭安進公司旗下的安進風投和賽爾基因等諸多機構。GNS Healthcare的目標是構建并調整其疾病模型。在最近的一系列試驗中,該公司詳細介紹了REFS在治療帕金森病等方面的潛力——多效性因素讓現有的帕金森病治療手段變成了撞大運。GNS Healthcare的首次試驗報告發表在2017年的《柳葉刀》醫學雜志上。
就帕金森病而言,基因缺陷觸發的互動網絡具有特定的形狀,而運動機能的分解是病情發展的最可靠指標。將帕金森病患者和控制組成員的基因數據輸入REFS幫助GNS Healthcare生成了100多個計算機模型,其作用是描述運動機能退化過程中可能發生的情況。這些模型可以發現此前未被人知曉的基因變異,而后者可能就是運動機能加速退化的原因。 |
At the dawn of modern genetic research, almost no one anticipated the enormous complexity in the biology of disease. Many researchers thought the genome would be a kind of instruction manual for the body. Pioneers such as Celera Genomics’ Craig Venter and Francis Collins of the National Institutes of Health were celebrated as “gene hunters,” a term that evoked crusaders scouring the globe for that one “silver bullet” gene that would explain—and facilitate a cure for—a given disease.
To some extent, these researchers found real treasure. Geneticist Nancy Wexler, for example, spent years in Venezuela compiling family trees of those affected by Huntington’s disease, a rare, inherited condition. Her work led to the discovery of the mutation in a single gene that predicts whether an individual will contract the condition.
But scientists soon realized that genetic maps were less like an instruction manual and more like the parts catalog you get with Ikea furniture. What’s more, researchers discovered other catalogs that added complex variables to the relationships between genes and disease—for example, the proteome, the proteins encoded by DNA, and the transcriptome, all the nucleic acids that convert DNA into proteins.
The morning-after disappointment has proved wrenching, as researchers learned that complex diseases, such as cancer and Alzheimer’s, didn’t yield to a single gene. (Even Huntington’s, its gene identified, has remained untreatable.) Today, Cohen and others see a link between the obsession with simplicity and a decline in drug discovery. That decline shows itself in the 1-in-10 success rate for FDA approval of new therapies; in spiraling costs for drug development (what a Tufts study recently identified as “the $2.6 billion pill”); and in the soaring prices of the few treatments that break new ground, such as the $475,000 cost of a course of treatment with Novartis’s leukemia drug Kymriah.
More recently, researchers have begun to grapple with biological complexity with the help of the science of networks. That science’s chief evangelist is Albert-László Barabási, a professor at Northeastern University whose 2014 book Linked popularized the notion that network theory can explain numerous fields, from fashion trends to sexual relations to disease. Barabási and others realized that disease is like a bad signal that moves through a network of connections from genes to proteins to cells to tissues, until all these “perturbations” manifest as the familiar symptoms of disease.
Complicated diseases are confluences of numerous effects, because pleiotropy means that any given protein can act at different points in the body. Startups like Pharnext assume that drugs can also be pleiotropic, acting on more than one protein and more than one interaction in the body at the same time. To find a drug combination capable of tackling complexity, the enormous power of machine learning, with its ability to spot patterns in data, must be wedded to a sense of the structure by which disease operates.
This, in turn, has required an evolution in the relationship between computer scientists and biologists. Newer machine-?learning approaches ingest vastly more data and can assemble hierarchies of information that let them go beyond correlation. Still, harnessing these “deep learning” neural networks into a structure that has any predictive power requires some elegant algorithm-building.
Colin Hill, CEO and founder of GNS Healthcare, is one of the builders. His company, based in Cambridge, Mass., has spent 18 years developing a computer system called REFS, which stands for “reverse engineering, forward simulation.” GNS has raised a total of $38 million over the years—from Amgen Ventures, the venture capital arm of the drug giant, along with Celgene and a variety of other investors—to build and fine-tune its models of disease. And in a recent series of trials, first published in 2017 in the medical journal The Lancet, GNS has detailed REFS’s potential when applied to a disease such as Parkinson’s—an ailment in which pleiotropic factors render existing treatments wildly hit-or-miss in their effectiveness.
With Parkinson’s, the network of interactions set in motion by defective genes has a particular shape to it, and the breakdown of motor functioning is the most reliable indication of its progression. Feeding the genetic data of Parkinson’s sufferers and a control group into REFS helped GNS generate over 100 computer models depicting what might be going on as motor function deteriorates. The models can uncover previously unknown genetic mutations that may contribute to the speedup of deterioration. |

|
但這只是第一部分。GNS Healthcare已經用這些發現在計算機上模擬了5000次隨機控制試驗,這些模擬都是為了預測采用不同治療方法時病情發展的速度。和尋求同樣結果的控制人體實驗相比,此類“速度測試”的經濟性要高的多。同時,已經和其他藥廠聯手的GNS Healthcare目前正在申請用類似的方法來對付糖尿病、肌萎縮側索硬化、多發性骨髓瘤和乳腺癌等等疾病。
作為CEO,科林·希爾說:“我們現在可以在電腦上創建、進行并展示患者及其病情,這樣就可以用不同的藥物和不同的護理管理干預方法逐一進行檢驗,然后找出哪種治療方法對哪些患者起作用。”
換句話說,這樣的模擬不光是要找到關聯,它還回答了如果……會怎樣的問題。如果我們用這種藥替代那種藥,那么這個病人的情況會如何?這種模擬以及就反向操作得出結論的能力是人工智能應用的一項近期成果。它正在變得越來越重要,而這在很大程度上要歸功于該公司的技術顧問朱迪亞·珀爾,后者長期從事人工智能研究,而且是加州大學洛杉磯分校的計算機科學教授。在去年出版的熱門書籍《為什么》(The Book of Why)中,珀爾描述了怎樣從簡單的模式感知中形成真正的智能,機器學習可以辨別出大量的模式,而真正的智能可以基于這些模式進行反向推理并得出結論。脫離了機制觀念,光是數據無法做到真正的洞悉。珀爾認為:“數據在因果關系方面非常笨。”希爾則更直接地說:“深度學習并沒有那么深。” |
But that’s just the first part. GNS has used those findings to create 5,000 different computer simulations of randomized control trials, each aiming to predict how fast the disease would progress with varying approaches to treatment. Such speed-?testing can be vastly more economical than seeking the same result through controlled human trials. And GNS, in partnership with other drugmakers, is now applying similar approaches to treatments for diabetes, ALS, multiple myeloma, and breast cancer, among other diseases.
“We now have the ability to create and construct, on the computer, representations of human patients and their diseases such that we can now probe, drug by drug, care management intervention by care management intervention, and say what treatments work for which patient,” says Colin Hill, CEO of GNS.
The simulation, in other words, is not just finding correlations: It is answering What if questions. What if we had given drug A instead of drug B to patient X? That ability to simulate and answer counterfactuals is a recent arrival in the practice of A.I. It owes its growing importance in large part to GNS’s technology adviser, Judea Pearl, a longtime A.I. researcher and professor of computer science at UCLA. In a popular volume published last year called The Book of Why, Pearl describes how true intelligence ascends from merely noticing patterns, which machine learning does in spades, to being able to express counterfactual reasoning about what would have happened, based on those patterns. Data alone, disconnected from any idea of a mechanism, doesn’t provide real insight. “Data is profoundly dumb about causality,” claims Pearl. Hill puts it more bluntly: “Deep learning is not that deep.” |
****
|
今年67歲的丹尼爾·科恩在突尼斯長大,那時他處在猶太人、基督教徒和穆斯林構成的多元化社會中,“他們用非常雅致而且和平的方式生活在一起”。他說這樣的經歷讓他形成了“事物是復合而非復雜”的概念。9歲時科恩隨家人移民到了巴黎,并開始熱情地學習彈鋼琴。后來他意識到,自己作為科學家的影響可能比音樂家大,這讓他立刻轉投醫學,但保持著同樣的熱情。他曾經是倫敦皇家交響樂團的客座指揮,夢想著率領該樂團演奏柴科夫斯基的《悲愴交響樂》。他開玩笑說:“交響樂團指揮、CEO和科學家體質都由同樣的基因控制著。”
此前科恩就曾經把基因學和技術轉化為藥物方面的成功。他同別人一起創立了Millennium Pharmaceuticals,這是一家腫瘤藥物公司,參與開發了多發性骨髓瘤治療藥物Velcade。
科恩樂觀地認為Pharnext能通過人工智能取得成功,但他也清楚這項技術的局限。谷歌的人工智能程序AlphaZero可以擊敗最頂尖的人類圍棋選手,而且沒有使用此前人類的任何圍棋知識。但就像科恩所說,圍棋有固定規則,而AlphaZero也完全明白這些規則。在生物學中,人們還沒有完全掌握相關規則,而且或許永遠也掌握不了,這在一定程度上要歸咎于多效性。 |
Daniel Cohen, now 67, spent his childhood in Tunisia’s heterogeneous society of Jews, Christians, Muslims, “living all together in a very elegant and pacific way.” He credits that experience for his taste for “things that are not complicated, but complex.” When he was 9, Cohen’s family immigrated to Paris, where he pursued the piano avidly. He switched to medicine once he realized he might have a greater impact as a scientist than a musician, but the passion has not left him. He has been a guest conductor at the Royal Philharmonic in London and dreams of leading that ensemble in Tchaikovsky’s Symphony Pathétique. “The predisposition to orchestra conductor, CEO, and scientist are all controlled by the same genes,” he jokes.
Parlaying genomics and technology into pharmaceutical success is something Cohen has done before. He was a cofounder of Millennium Pharmaceuticals, a U.S. oncology-drug maker that helped develop the multiple-myeloma treatment Velcade.
Cohen is bullish that Pharnext can be successful with A.I., but he is also aware of the technology’s limitations. Google’s ?AlphaZero, an A.I. program, was able to beat the world’s human masters at the Chinese strategy game Go, without using any prior human knowledge. But as Cohen points out, Go has a finite set of rules, which AlphaZero knew completely. In biology, thanks in part to pleiotropy, the rules are not fully known—and may never be. |

|
但設計周密的人工智能已經讓Pharnext能夠圍繞已知規則建立模型并做出相應的選擇。在10000種已知藥物中,該公司的發現模型用其中的2000種進行搭配,這些藥品的專利已經過期,而且已經得到推廣,或者說其療效和安全性都足以向公眾銷售。
為開發腓骨肌萎縮癥治療藥物,Pharnext首先用了約一年時間來構建這種疾病的網絡模型,或者說和GNS Healthcare帕金森病圖譜類似的框架,從而說明相關基因突變如何引發了一連串神經和肌肉問題。基于這樣的機理,計算機模型挑選出了57種候選藥物,分別針對這一連串問題中的各個點。Pharnext對這些藥物逐一進行體外測試,再從中選出22種在老鼠身上試驗的藥物,最終形成由三種藥物構成的組合進行臨床試驗。三期臨床最近公布的積極結果證明PXT3003正在這一連串問題的多個點上發揮作用。
科恩說,如果沒有人工智能模型,預臨床測試需要的時間就會多出好幾年,而不是Pharnext所花的三年,“從2000種藥物[中著手],我就可以得出所有可能的組合,即10億種可能性”來進行體外測試。這個“處方”中有無數的錯誤測試結果和死胡同,意味著好幾年的挫折——現在這些都得以避免。 |
But thoughtfully designed A.I. has enabled Pharnext to build models around the rules that are known and make choices accordingly. Out of the universe of 10,000 known drugs, the company’s discovery model takes in an assortment of 2,000 that are both out of patent and “marketed”—that is, already judged both therapeutically effective and safe enough to be sold to the public.
To develop its CMT drug, Pharnext first spent about a year assembling its network model for the disease—a framework comparable to GNS’s Parkinson’s map, showing how nervous and muscular problems “cascade” from the relevant gene mutation. Based on this mechanism, the computer model arrived at a short list of 57 candidate drugs that addressed various points in the cascade. Pharnext tested those drugs one by one in vitro, generating a shorter list of 22 to be tested in mice, which finally yielded the three-drug combination that went to human clinical trials. The recent positive Phase III results confirmed that the PXT3003 cocktail is acting at various points in the cascade.
Without the A.I. model, many more years of preclinical testing would have been required beyond the three years it took Pharnext, says Cohen. “With 2,000 drugs [to start with], I could produce all possible combinations, a billion possibilities” to test in vitro. That’s a recipe for countless false positives and dead ends—years of frustration, for now forestalled. |
****
|
Pharnext在巴黎上市,去年10月公布上述三期臨床結果后其股價以上漲了一倍多。10年來,該公司已經在研發上投入約1.2億歐元(約1.35億美元)。就制藥行業的標準而言,這個數字著實不多。Pharnext尚未盈利,但分析師估算,如果PXT3003上市,該公司的收入將從2020年開始飆升。2018年Pharnext實現收入900萬歐元(GNS Healthcare未上市,沒有披露過開支或收入)。
除了投資者可能獲得成功,Pharnext和GNS Healthcare的進步還為人工智能以及與之共同成長的藥物學指明了發展方向。理解因果關系以及反向探索問題的能力是人工智能用戶長期以來一直想跨越的門檻。隨著它們掌握并控制眾多讓人眼花繚亂的變量,這些初創公司的計算機模型正在朝著這個方向邁進。
就連疾病的基本定義都可能發生改變。隨著科學家不斷加深了解,這些定義已經變得過于簡單。學術期刊《Bioinformatics》在去年的一項研究指出,癌癥中的基因變異存在“根本性差異”,而這對癌癥治療產生了不利影響——看似是同一種疾病,或者同一類疾病,而實際上患者之間的相似之處寥寥無幾,區別卻有很多。人體內的任何變化都絕不等同于單個基因的“開/關”引發的一系列癥狀,弄清楚了這一點,科技就可以幫助從業者克服藥物開發的復雜性。
最后但同樣重要的是,這些人工智能推動的工作從經濟角度帶來了一絲希望。在這個時代,藥物開發成本已經成為令人畏懼的障礙,智能算法則有可能在某一天讓醫藥利益相關方從投入藥物研究的數萬億美元中獲得更多的價值。科恩判斷,理論上,通過老藥新用,“人們就不需要再設計[新]藥物。我的感覺是可以用50種藥物來治療所有的疾病。”這就意味著另一個詞的定義也要變了,那就是“發現”。(財富中文網)
本文另一版本刊登在2019年4月出版的《財富》雜志上,題為《在老藥中找到新療法》。 譯者:Charlie 審校:夏林 |
Pharnext’s shares, which trade on the Paris stock exchange, have more than doubled since October’s Phase III results announcement. The company has spent about 120 million euros ($135 million) over the past decade on research and development—a very modest figure by pharma standards. It has never made a profit, but analysts estimate that if PXT3003 reaches the market, revenue—9 million euros in 2018—could soar starting in 2020. (GNS Healthcare is privately held and does not disclose spending or revenue.)
Beyond possible victories for investors, the advances at Pharnext and GNS point the way to A.I.’s growing up—and pharmacology along with it. The ability to reason about causality, and to explore counterfactual questions, is a threshold that users of artificial intelligence have long sought to cross. The computer models at these startups are making a foray in that direction as they manage and tame a bewildering number of variables.
Even the underlying definition of disease may evolve. As scientists are learning, these definitions have been overly simplistic. A study in the journal Bioinformatics last year noted that attempts to treat tumors are hampered by the fact that genetic mutations in cancer are “fundamentally heterogeneous”: What appears as one disease, or class of disease, in fact contains few commonalities and many differences from patient to patient. As it becomes clear that what’s happening in any body differs sharply from the “on/off” model of one gene turning on a set of symptoms, technology can help drug developers wrestle with the complexity.
Last, but hardly least, these A.I.-driven efforts offer a glimmer of economic hope. In an era in which the cost of drug development is a daunting obstacle, smart algorithms may someday enable medical stakeholders to derive more value from the trillions of dollars that have already been spent on drug research. In theory, with repurposing, “you don’t need to design [new] drugs,” Cohen avers. “My feeling is that with 50 drugs, we can treat everything.” That would mean changing yet another definition: the meaning of “discovery.”
A version of this article appears in the April 2019 issue of Fortune with the headline “Finding New Cures in Old Drugs.” |













